Baricitinib as potential treatment for 2019-nCoV acute respiratory disease
by Peter Richardson, et al, The Lancet
Given the scale and rapid spread of the 2019 novel coronavirus (2019-nCoV) acute respiratory disease, there is an immediate need for medicines that can help before a vaccine can be produced. Results of rapid sequencing of 2019-nCoV, coupled with molecular modelling based on the genomes of related virus proteins,1 have suggested a few compounds that are likely to be effective, including the anti-HIV lopinavir plus ritonavir combination.
BenevolentAI's knowledge graph is a large repository of structured medical information, including numerous connections extracted from scientific literature by machine learning.2 Together with customisations bespoke to 2019-nCoV, we used BenevolentAI to search for approved drugs that could help, focusing on those that might block the viral infection process. We identified baricitinib, which is predicted to reduce the ability of the virus to infect lung cells.
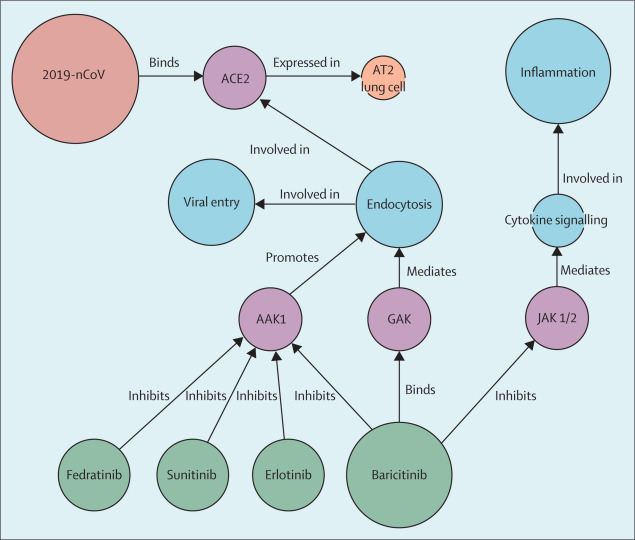
Most viruses enter cells through receptor-mediated endocytosis. The receptor that 2019-nCoV uses to infect lung cells might be ACE2, a cell-surface protein on cells in the kidney, blood vessels, heart, and, importantly, lung AT2 alveolar epithelial cells (figure). These AT2 cells are particularly prone to viral infection.3 One of the known regulators of endocytosis is the AP2-associated protein kinase 1 (AAK1). Disruption of AAK1 might, in turn, interrupt the passage of the virus into cells and also the intracellular assembly of virus particles.4

Of 378 AAK1 inhibitors in the knowledge graph, 47 have been approved for medical use and six inhibited AAK1 with high affinity. These included a number of oncology drugs such as sunitinib and erlotinib, both of which have been shown to inhibit viral infection of cells through the inhibition of AAK1.5 However, these compounds bring serious side-effects, and our data infer high doses to inhibit AAK1 effectively. We do not consider these drugs would be a safe therapy for a population of sick and infected people.




