Stanford Researchers Unveil New Ultrafast Charging Aluminum-Ion Battery
by Robert Fares, Scientific American
Last week, Stanford University researchers unveiled a new aluminum-ion battery chemistry with the unique ability to charge or discharge in less than a minute.
It is also the first aluminum-based battery to achieve an operating voltage sufficient for common applications and last longer than a few hundred charge-discharge cycles.
What’s Inside the Aluminum-Ion Battery?
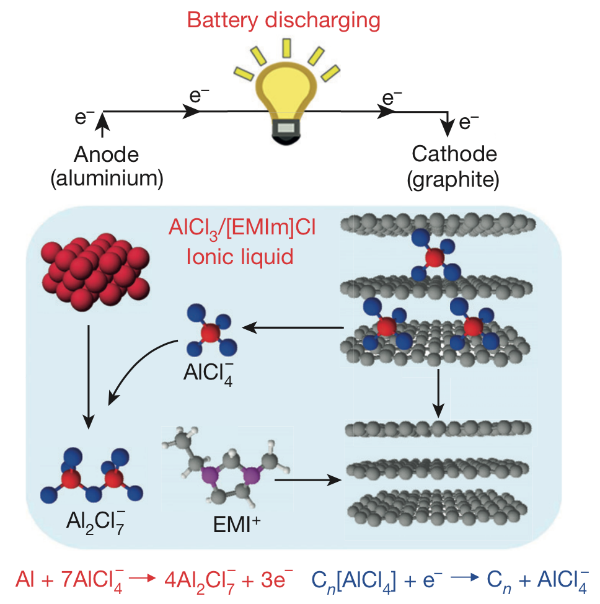
To store energy, a battery requires two materials with an electrochemical voltage difference between them and an electrolyte that impedes the flow of electrons but enables the flow of ions between the two materials.
The aluminum-ion battery introduced last week uses simple aluminum metal in its negative side (anode) and a specialized three-dimensional graphite foam in its positive side (cathode). The positive and negative sides of the battery are separated by a liquid electrolyte of 1-ethyl-3-methylimidazolium chloride and anhydrous aluminum chloride. This electrolyte was selected because it contains mobile AlCl4- ions, which are exchanged between the two sides of the battery as it charges and discharges.




